| Metalic bonds | many metals have a low value of E |
| Ionic bonds | ΔE: > ±1,6 |
| Covalent bonds
- covalent, non polar - covalent, polar |
0 < ΔE < ±1,6
0 < ΔE: < ±0,4 ΔE: > ±0,4 |
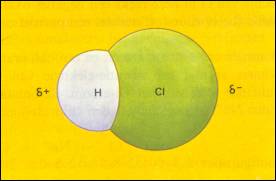
| CS2 (ΔE = ±0) | CO2 (ΔE = ±1.0) | H2O (ΔE = ±1.3) |
| Covalent bonds | Covalent bonds | Covalent bonds |
| Non polar molecules | Non polar molecules | Polar molecules |
| There is no dipole | There is no dipole | There is a dipole |
| There are no δ+ and δ- | There are δ+ and δ- of which the central points do overlap |
The central points of δ+ and δ- do not overlap each other (remain at a distance) |
|
S=C=S
|
O = C = O
δ- δ+ δ- |
δ+
δ+
H H \ / O δ- |