Activation energy
Most chemical reactions need some 'help', kind of starting motor, just to start up the reaction.
Even a reaction of petrol with Oxygen needs a bit of such activating energy: just a spark or a match is enough in this case.
Other reaction may need a lot of activating energy, like the formation of ammonia from the elements Nitrogen and Hydrogen.
All kinds of energy may help start the chemical reaction: light, movement, heat, electricity.
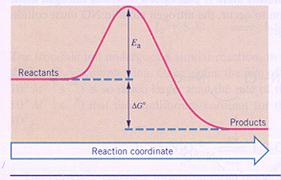
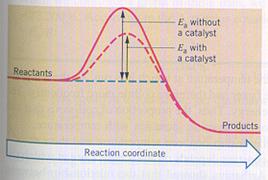
A catalyst can have a lot of influence on the needed activating energy. There are reactions that fail without catalyst.

