How to influence chemical reactions
If reaction rate depends on the number of effective collisions, the next question is:
- How to influence the number of collisions?
- How to influence the effectivity of the collisions?
Factors that influence the number of collisions:
- The number of present particles depends on the concentration, or (with gases) on the pressure. The more particles, the more chance to a collision.
Att: during a reaction the number of reacting particles automatically will decrease, so, of course the number of collisions will decrease, or: the reaction rate will decrease with time
- The character and the situation of the reacting particles.
How are they present in the reaction? Are the divided in extremely small particles, or still stony? Have they little or great energy? Are they well mixed? etc.
- The temperature of the reactants. This has immediately influence on the movement: higher temperature, more movement, more chance to collision.
Att.: We only talk about concentration when the division of the particles is homogeneous. In the case of heterogeneous substances, we do not speak of concentration.
In the rate formulas, heterogeneous substances are simply filled out with 1.
Factors that influence the effectivity of collisions
- The presence of a catalyst. That one makes that the collision between particles is less by chance, less by accident; a catalyst accompanies, directs collisions.
The particles collide exactly on the right spot, for example, thanks to a catalyst that has a big influence this way (see also biocatalysts)
- Again the temperature. Not only the movement of the particles will increase, but also their energy. And that can provide with higher effectivity.
att.: Every chemical reaction needs a minimum energy to start. Thus you can 'freeze' reactions:
As quick as possible lower the temperature under that minimum; then the rate comes at once to zero mol.sec.
In the synthesis of ammonia according to Haber Bosch, the catalyst will help the step that divides N2-molecules into two atoms of N.
After that, a N-atom reacts with H2, etc.
Not every step has the same rate.
Extinguish
There are a number of ways to extinguish a fire:
- cooling down until the temperature is under its minimum reaction temperature.
- removing reactants, or prohibit the arrival of reactants
- Add a helpinbg substance that itself cannot burn
(Bio)catalysts
In diagrammes you may see the chemical energy on the vertical axes and the course of the reaction on the horizontal axes:
Ozon + atoms of Oxygen normally form Oxygen, with help of the catalyst Clorine atoms.
Without clorine their is the need of an intermediate product with much energy (O3---O); the formatiuon of that one costs a lot of energy.
The atom of clorine can make a different intermediate product that costs less energy.
Temperature
In general you may say that the increase of temperature will increase the reaction rate.
Every 10 degrees increase
will double the reaction rate.
(kind of general rule)
Inrease of temperature means automatically: more movement of the present particles;
and this means more collisions between the reacting particles and more energy for each.
Concentration; adding or removing substances
The following diagramme shows the concentrations of the reactants and products of a process that is about to reach equilibrium:
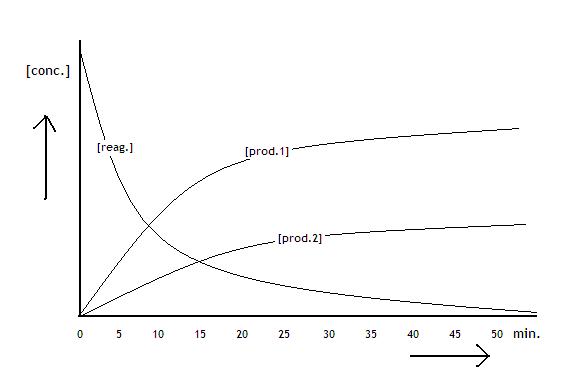
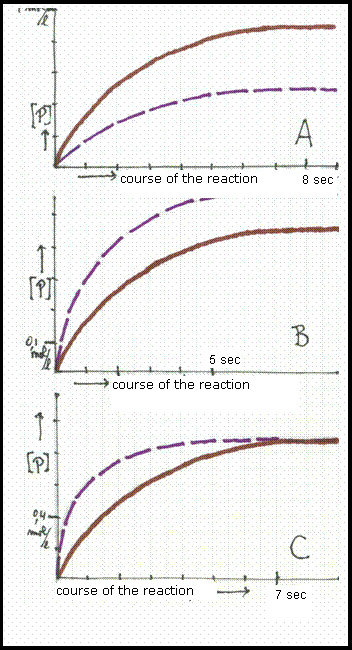
The three diagrammes show graphics:
concentration of the product in mol/l versus course of the reaction in seconds.
Two graphics differ only in temperature, in such a way that the broken line shows the situation at the lowest temperature.
The reaction rate depends always on the concentration of the homogeneous substances. It will have immediate concequenses when changing one of the reactants.
At adding one of the reactants, the rate must increase immediately (for some time!).

