Distillation of (l) + (l)
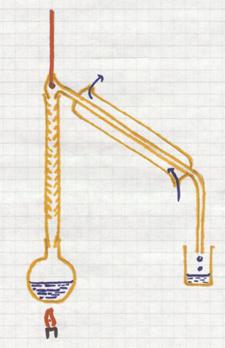
This is a separation method based upon the difference in boiling points of liquids.
If you heat a mixture of liquids, the liquid with the lowest boiling point will boil first, escape first (evaporate) from the mixture.
The vapor enters the condensation tube with cooling. There this component will condens and collected.
The other components, with higher boiling points, remain behind until reaching higher temperatures.
Good controll of the temperature is needed during the whole process.
example: you can distill wine, where alcohol with the lower boiling point (78ºC) will escape. the water will remain behind
