Thin Layer Chromatography
Cromatography often is applied in chemical laboratories:
a mixture (i.e. with various particles) passes a fixed layer (paper, gel of aluminiumoxyde, or other porous material).
The different particles of the dissolved mixture will be more of less absorbed by that fixed layer (dependent, for example, of polar properties).
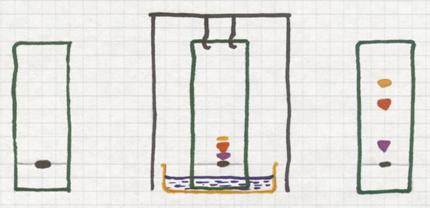
If you use a black fineliner drawing a line on e piece of filter paper, and then you allow a liquid to pass this paper from bottom up, then you will see that the line can go up with the liquid, even that the ink was composed of different color components.
One color will go better, faster with the liquid than the other.
Each color component in the ink continuously must chose: to stay abosorbed in the paper or to dissolve in the moving liquid and dislocate.
These to tendencies, staying in de fixed layer and dissolving in the 'walking' liquid can be called: the affinity of the component to the phases.
The above described separation of colorants in a sheet of filter papaer belongs to the thin layer chromatography.
The paper is supposed to be the stationary phase (or contains it).
Another option is that a thin layer of solid (fixed phase) is put on an Aluminium strip. the mobile phase is here always(l); it passes the pores of the fixed phase (by diffusion).
paper chromatography
Put on a piece of filter paper a small dot of black ink (wich certainly is a mixture of various colours).
Choose a 'mobile liquid' and put the lower end of the paper in that liquid. Through capilary activity, that liquid goes up, passes the spot of ink. At that moment, the liquid moves the different coloured components up with different rates.
Thus the less soluble component will probably stay without moving at all. But a component that dissolved reasonably in the liquid will be moved up. That's how you separate the components.
At the end you will see different spot in various colours.
