The use of electrodes
Below you see a schema of a simple chemical cell, voltaic cell, with copper/zincum electrodes.
Consider well this scheme:
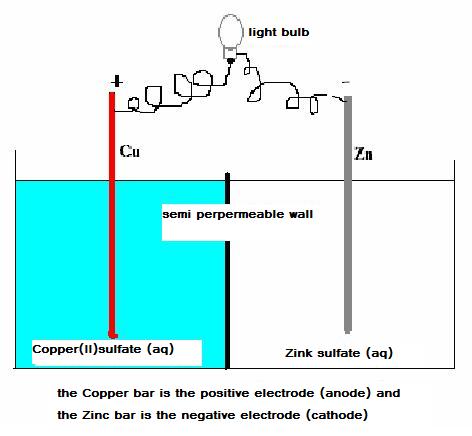
In the whole complex you can discover a sufficiently strong oxydator (Cu2+) and also a rather strong reductor (Zn)
The (spontaneous) reactions dominating the process are:
Zn(s)  Zn2+ + 2e-
Zn2+ + 2e-
and
Cu2+ + 2e-  Cu(s)
Cu(s)
You should know that realising a redox reaction at the surface of an electrode almost always cost a certain activating energy.
Such a reaction must me started up.
Certainly if gases are formed during this reaction, this activating energy can be considerable.
Indirect redox reactions only take place if, between the electrodes, there is an environment conductive for electric current (meaning: charged particles must be present that can freely move around, like ions of dissolved salts).
You can also say that the current circuit must be closed; part of the circuit are the conductive metal wires and the electrodes with free electrons, and the other part is the solution or melt with free ions.
Those free inons can freely move between the postive and the negative electrode.
To mak this movement possible, the electrode compartments must have a contact.
This can be a semipermeable (porous) barrier, or else - if the two electrode compartments do not have direct contact - those compartments must be connected via an ion bridge, a salt bridge. This is some kind of tube filled with salty gel (a gel with ions).

An electrode can have lack of electrons;
than the electrode is positive: anode
An electrode can have extra electrons;
than the electrode is negative:cathode
Cathode-Negative // Anode-Positive (CNAP)
Electrodes are made of conductive materials: mostly a metal, maybe graphite.
Those substances contain free electrons in a metal lattice. These free electrons can freely move within the electrodes, coming from the negative and going to the positive electrode (one-way traffic!)
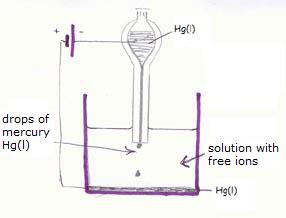
A very special one is the 'liquid electrode':
Inert and participating electrodes:
If the electrode material is of the type: 'very weak' (as reductor or as oxydator he is nothing), than this electrode will be INERT.
The only activity is transporting electrons, but it does not take part in the real redox reaction.
Examples: gold, platinum, graphite.
On the other hand, we use electrodes made of materials that really participate in the redox reaction, for example, in the case of an iron or zincum electrode.
An iron bar can very well serve as an electrode, but will send iron ions into the solution, while electrons remain behind in the barr.
Really, after some time such an electrode will slowly be consumed; it becomes thinner and disappears.
The iron suffers the following half reaction:
Fe  Fe2++2e-
Fe2++2e-
where:
- Fe is the iron of the electrode
- The Fe2+ -ions go into the solution to move there freely in the direction of the negative electrode.
- The electrons staying behind in the iron electrode will participate in the electron transfer and the electron transport.
A participating (so not inert) electrode always is made of neutral, non noble metals; they serve as reductors, or: they can donate electrons.
The word "electrode compartment" has been used a few times now;. that is the space directly around an anode or cathode, its surface included.
There the half reactions of the indirect redox reaction take place.
The Cathode and anode compartments may not be separated physically, and everything takes place in one solution or liquid.
To keep the situation equal in all places, stirring of the solution is needed continuously.
Another option is: the two processes occur in separated compartments, but connected with semiporous barriers of with salt bridges.
Without one of these, there is no closed circuit and current is impossible (nor any transfer of electrons)
In the case of a salt bridge, take care that the ions in that salt bridge themselves do not participate in the redox reaction.
A choice pro or contra separated electrode compartments has all to to with what you aims with the redox reaction in charge.
Keeping apart mostly aims to keep de products separated.
These product could possibly react (when in contact), and - for example - produce a precipitate or a gas.
Onthe other hand, someone can also wish that happen.
A solution (or a molten substance) conducts electricity only under the condition of the presence of charged and movable particles (ions in this case).
The conductibility has everything to do with the movability of those ions:
The more movalbe, the better they conduct.
That movability depends on some factors:
- how many ions are present?
- are these ions big or small or hidrated?
- Somtimes an ion (certainly the H+-ion in water) has special techniques to improve their movability.
att.: a metal or graphite cannot serve as a salt bridge.


