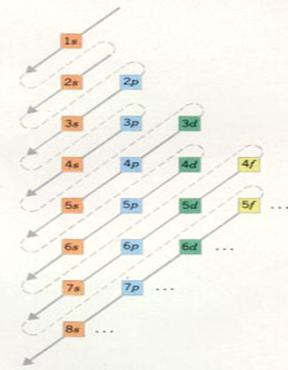
| Main level 1 has only one sublevel, | of the type s | with a maximum electron number of: | 2 |
| Main level 2 has 2 sublevels | of the type s and p | with a maximum electron number of: | 2 and 6 |
| Hoofschil 3 has 3 sublevels | of the type s, p and d | with a maximum electron number of: | 2, 6 and 10 |
| Main level 4 has 4 sublevels | of the type s, p, d and f | with a maximum electron number of: | 2, 6 , 10 and 14 |
The main levels 5 to 7 - theoretically - could be divised in 5, 6 and 7 sublevels.
But no such big atoms exist; nature did not realise them. so: |
|||
| Main level 5 divides itself in 4 sublevels | of the type s, p, d and f | with a maximum electron number of: | 2, 6, 10 and 14 |
| Main level 6 divides itself in 3 sublevels | of the type s, p and d | with a maximum electron number of: | 2, 6 and 10 |
| Main level 7 divides itself in two sublevels | ofthe type s and p | with a maximum electron number of: | 2 and 6 |