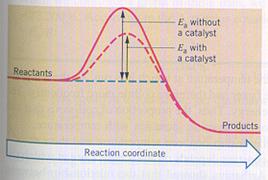
Energy diagrammes
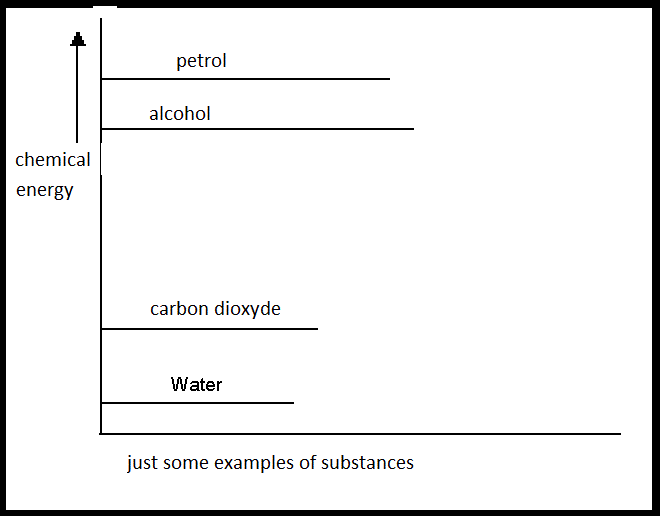
If petrol reacts with Oxygen (two energy-rich substances), thr products are water and carbon dioxyde (two energy poor substances)
The system loses a lot of energy during this combustion. And energy does not disappear just like that, because energy is never lost.
This energy goes outside the system; in this case in the form of heat.
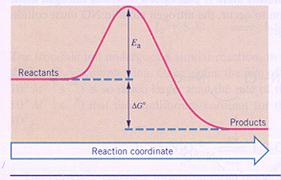
The difference in energy between products and reactants is sometimes called: the reaction energy with a symbol: ∆H.
If energy escapes from a reaction system, ∆H will have a negative value. Then we have an 'exothermic' reaction.
If energy is captures by a reaction system, ∆H will have a positive value: an 'endothermic' reaction.