Regulation of the enzym activitity
Non catalised reactions, needing for example hours of cooking, even in the presence of strong acid or base, sometimes can occor in a couple of seconds at a decent temperature if the right enzym is present, and at the optimal pH and temperature.
The enzyms perform as catalysts by lowering strongly the needed activation energy of this specific biochemical reaction.
The lowered activating energy allows the process to occur at body temperature and at high speed.
There are different ways to regulate the enzym acitivity:
Different enzyms are not immediately ready to function, but have a "pre-enzym phase", or: a zymogene phase.
This can be necessary to protect a vulnerable enzym.
It is also possible that an enzym is only needed in the case of accidents.
In the stomach we have 'pepsinogene', dat can produce pepsine enzym at a low pH. If it is produced, the pepsine cannot go back. The pepsinogene loses a part of amino acid chain of 44 amino acids.
The product itself can (if it has reached high concentrations) react back and thus forbid an enzyme to continue its activity.
If we know the enzym, specific substances can be added that can influence the enzym activity (inhibitors = molecules with a structure more or less similar to the normal substrate, but not exactly the same).
Such an inhibitor can make the enzym (temporarily) inactive. Another way of regulation.
In fact we talk here about drugs, that - for example - are used in chemo therapy, forbidding the metabolism of tumors.
Some enzyms have, apart from an action centre, another place where certain groups can connect: a so called allosteric centre.
Substances that fit in such an allosteric centre, can change the threedimensional shape of the enzym, including the action centre, and so change the specificity of this enzym, and with that, its activity.
This can have a positive influence (an more active enzym) but also a negative influence.
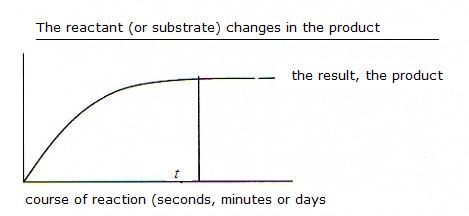
Most biochemical reactions are also reactions in equilibrium.
That means that the formation of the product, after some time, will reach a maximum rate at the moment that the equilibrium has been reached.
Without catalyst (see module 7), this can take an awful time: hours of even days or never.
In the presence of a catalyst (an enzym) the equilibrium is reached much faster.
This does not mean that you will obtain more product; but yes, the product was formed faster, maybe within seconds.
The enzym, the biocatalyst, mostly speeds up the chemical proces with a factor of 106 or more.

The substrate forms a complex with an enzym. This complex is called: the transition state. The substrate changes into a product.
After that, the product and the enzym separate.
S + E  ES
ES  P + E
P + E
a b
The formation of the comples (a) also is an equilibrium; the last reaction (b) is not.
The enzym speeds up the realisation of the equilibrium, but does not change the equilibrium itself. Only the formation rate of the product.

