The phases (s) (l) (g) (and (aq))
Have a look: film.
att.: Talking about phases, officially (aq) is not a phase.
Below a scheme that shows models of each phase on the level of particles. You can find that also in the tables.
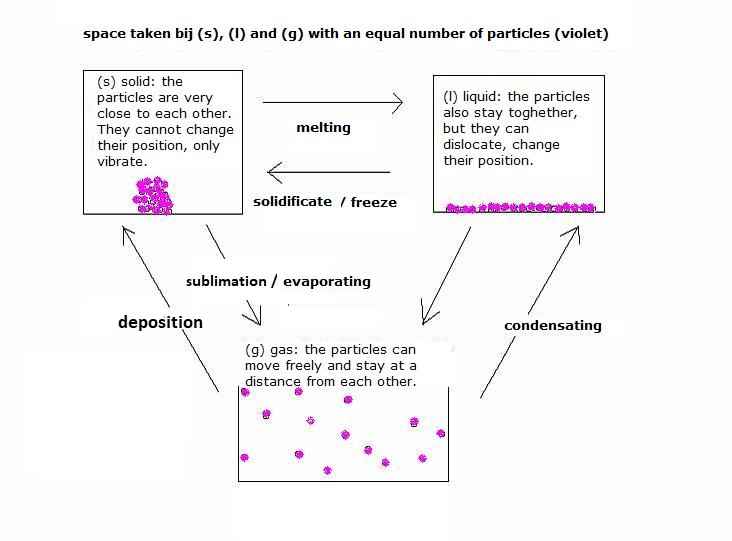
In solid state, the particles are positioned on a fixed place; they cannot dislocate. They cannot move around. But yes, they can (and often will) be in some vibration, but are not mobile. The vibrations depend on the temperature. At very low temperatures, the vibration can even stop.
Normally al those particles toghether make a regular pattern, a lattice. You can observe that outside: a cristal structure. But there are solid substances without cristal structure: amorphic. Examples are: glass, plastic and gel.
In the liquid phase the particles have a certain movability; they can deslocate, turn around each other. Just like all particles, they also have a certain vibration movement. The whole movement depends on the character of those particles (for example: being big or small) and on the temperature (cinatic energy).
The particles in a liquid do not form stiff structures, no cristals, but do remain close, as close as possible like in a solid, thanks to the mutual attraction. It is wrong to think that a liquid is something in between solid and gas. Liquids and solids have a comparable density. The fact that the particles can move, around each other, causes the liquid character.
In the gasphase the particles are completely separated from each other; they move separately; they have all freedom for that, but dependent of the character of the particles and the temperature.
Regularly the gas particles collide mutually (or they collide against the walls of the gas holder) and thus change there movement direction. If they have no longer enough energy, for example at cooling, than a collision could end with the staying toghether of the particles → liquid or solid.
N.B. About liquids there is this misconception:
In a liquid, just as in a solid, the particles stay close toghether.
The difference with a solid is that the particles in a liquid are not in e fixed position, a lattice, but can move around each other; they have enough cinetic energy for that.
You could also say: the mutual attraction is not enough to make a lattice. Taking away energy, cooling for example, could cause a lattice anyway. Heating, so when particles aquire more energy, than it can happen that they start to remove from each other (go to gas phase).
In a liquid the particles are that close toghether that there is no option (as in gases) to put them more togheter and getting smaller volume.
(aq) we use many times in chemistry, but is no phase. It means a mixture where a solute is dissolved in water.
NaCl(aq) means: a solution of salt in water.
