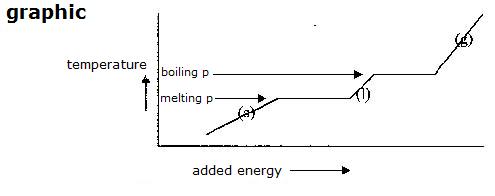
Energy and change of phase
When adding energy to a substance, that substance normally wil get hotter. The particles receime more movement!
Only if that increase of energy is accompanied with phase change, then (temporarily) something extra is happening:
during this change engery is used to remove particles further from eacht other, or the lattice of the substance is destroyed.
The temperature does not change during this change of phase as long as the two phases are still there.