The volume of gases
In particular we look at the volume of 1 MOL of gas. Because that is special in the case of gases. (and not with (s) or (l))
Any gas at the temperature of 0oC and a pressure of 1 atm. has the volume of 22,4 liter per MOL (= molar volume of gases).
Att.: Every gas has the same molar volume, but mind: not the same molar mass!!
It does not matter with what gas you are dealing, the volume that it occupies is always determined by the temperature and the pressure of that gas,
wether the molecules are big or small.
We apply that with the following formula:
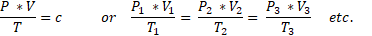
The temperature T is given in Kelvin!! The pressure in Nm2. The volume in m3
The constant c is: n x R, where:
n = the number of MOL and R = 0,0821 J x mol-1 x K-1