The ionic lattice
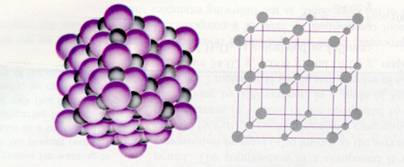
The bigger purple spheres represent the negative ions, and the grey smaller spheres are the positive metal ions.
Substances built up of ions form IONIC LATTICES.
The different ions will pack neatly, in a very regular way.
positive ions are surrounded by negative ions, and negative ions are surrounded by positive ones. Alle because of the attraction forces of course).
Those lattices are strong, because of all those attraction forces.
It is good to know already that most substances with an ionic lattice are called: salts.
All substances built up of ions, do not form molecules.
Molecules are treated further in this module, but it is good to know already that molecules are built up of connected atoms, not as ions.
Molecules are neutral particles.
Also substances with ionic bonds are neutral, but only because there are equal amounts of positive and negative charges.
Positive and negative ions always join toghether in such a way that in total a neutral substance is made: with the same positive and negative charge.
The positive and negative ions join in a certain fixed proportion in unbelievable large amounts, and are very nice arranged toghether in the
IONIC LATTICE.
The proportion of pos and neg ions is indicated with numbers right under, as if it were a molecular formula.
But it is not a molecular formula. this is a Empiric Formula, of a Proportion Formula.
Att.: It looks as if there is no difference between proportion formulas and molecular formulas.
