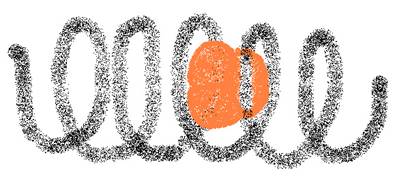Carbohydrates, saccharides, sugars
Structures of the saccharides
A simple division of the saccharides:
- Monosaccharides: glucose, fructose, galactose(C6H12O6)
- Disaccharides: saccharose (sugar), lactose, maltose (C12H22O11)
- Polysacchariden: amide (starch), amilose, cellulose( (C6H10O5)n)
Structures:
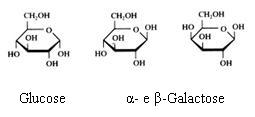
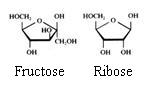
Monosaccharides
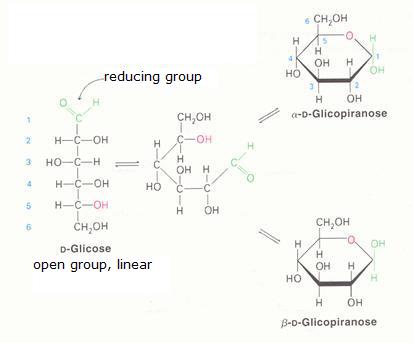
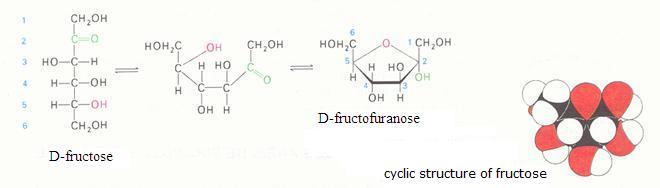
The monosaccharides can occur in a linear or in a cyclic configuration (above you only see cyclic forms).
Below you can see how glucose can have the linear and the cyclic form:
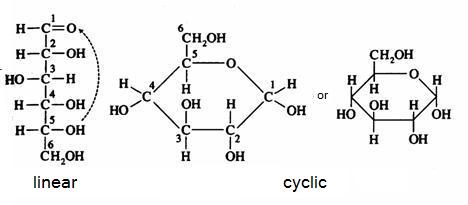
The linear structure can easily be oxydised, with a weak oxydator; often we use for that:
Ag+ ammonia-silver-solution = reagets of Tollens. Another one, Fehlings' reagent, also acts very fine (Cu2+)
The cyclic configuration is not easily oxydised (there is no =O bond available).
In practice the two structures, the linear and the cyclic, in a solution, are in an equilibrium with each other, so the linear structure is than always present and always ready to suffer oxydation.
Than the linear structure disappears in that process, the equilibrium will shift from the cyclic form tot the linear form (that continues to suffer oxydation), and at the end, all the monosaccharide is oxydised.
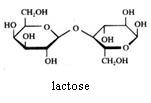
Disaccharides
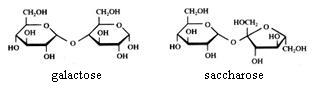
Saccharose cannot be oxydised with Ag+ or with Cu2+.
Maltose and lactose can be oxydised with Ag+ or with Cu2+.
Polysaccharides
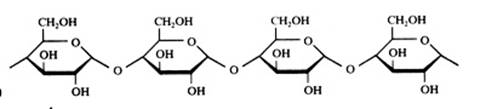
Amide, starch, cannot be oxydised with Ag+ or with Cu2+.
Amide has a helix structure when in natural form
Than it also creates with I3--ions a dark blue complex.
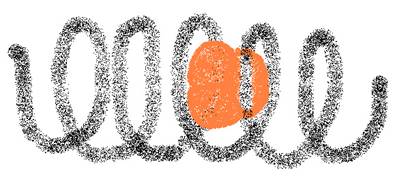
Fehlings' Reagent
CuSO4(aq) + 2NaOH(aq)  Cu(OH)2(s) + Na2SO4(aq)
Cu(OH)2(s) + Na2SO4(aq)
Cu(OH)2(s)  Cu2+ + 2OH-( = equilibrium 1)
Cu2+ + 2OH-( = equilibrium 1)
Cu2+ + e-  Cu+
Cu+
Adding of Sodium tartrate: the tartrate ions attrect and crab the Cu2+-ions from equilibrium 1; therefore the precipitate Cu(OH)2 will disappear; at the same time appears a transparant solution with dark blue colour = Fehlings' Reagent (FR).
FR in basic environment has dissolved Cu2+-ions capable to oxydise a reductor (for example the aldehyde group), capturing an electron and creating an ion Cu+.
These ions Cu+, in a secondary reaction with OH- will form the orange red precipitate Cu2O(s.
This recognition reaction can be used as proval of the presence of monosaccharides.
Aspartame is an artificial, non-saccharide sweetener, used by people that try to loose weight.
They want to prevent that they become fat by eating too much sugar (or they have other reasons).
Fact is that sugars contain a lot of energy. In stead of sugar in the coffee, we can take substitutes like aspartame; but there are others.
Many sugar containing products (for example jam and coca cola) have also their diet products, i.e. without sugar, but with the substituting sweeteners.
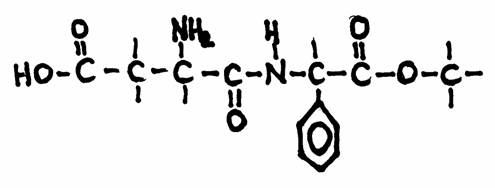
The secret of aspartame is that it leaved the body as it entered it, without participating in the metabolisme, without any energy impact, without any fat making.
But a warning is needed: some sugar substitutes can be carcinogeneous, not healthy.