The Carbon atom
For several reasons the Carbon atom is very special:
- every Carbon atom can make four bondings, which is more than other atoms can.
- The Carbon atom connects easily to other Carbon atoms. Silicon can do that more or less, but normally 'atoms don't like that'.
Most atoms prefer to connect with atoms of another type. There is a preference rule for connecting different elements.
Except for Carbon however.
Another thing is that the four bondings can be different in character: simple, double, triple. Besides that they can be aliphatic or aromatic.
To understand all this, you need to go into the story of 'orbitals'.
Two orbitals of Carbon atom are important for the making of bonds: the sublevels 2s and 2p.
These orbitals can cooperate in various ways (when making bonds). They also can mix and thus form 'hybrides' :sp3, sp2, sp.
Below a try out to make this clear:
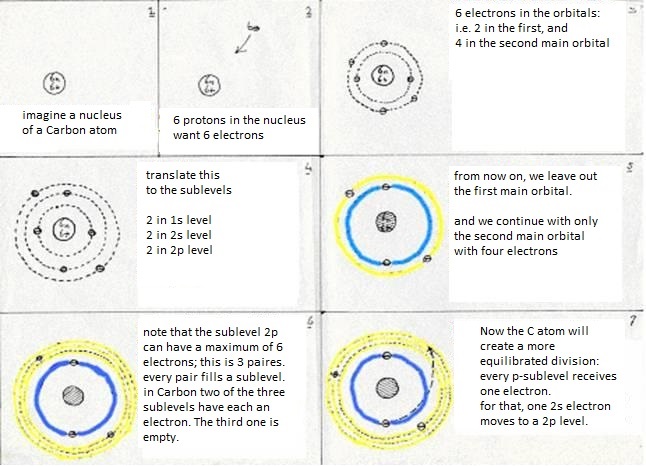
The sublevels of Carbon are: 1s2 2s2 2p2
The first main level 1s2 suffers no change at all, whatever the Carbon atom does.
But in the second main level occurs a kind of reorganisation:
The four valency electrons (two s and two p) have found a way to come to a more stable situation:
- first: a 2s electron becomes a 2p electron (so goes a bit further away from the nucleus, which costs energy)
- then: 2s1 becomes 2p3 (this change also costs energy)
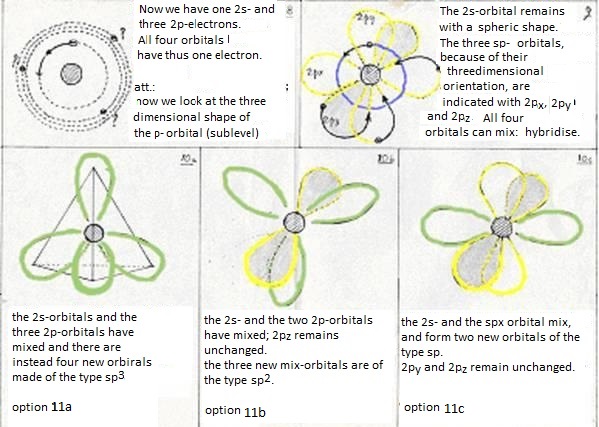
- at last: the four levels will now mix and form hybrides. Or: one 2s-orbital and three 2p-orbitals participate in this hybridisation.
They form four new orbitals of the type sp3 (here energy is set free = exothermic).
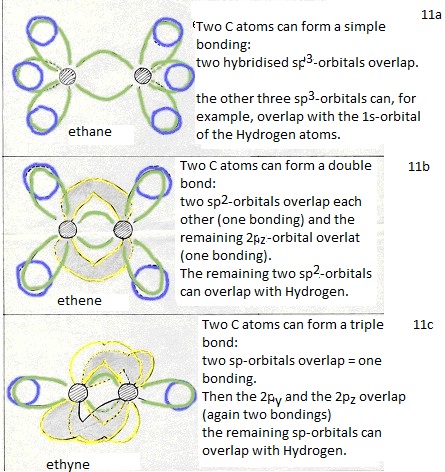
These four new and equal orbitals are responsible for the four (similar, equal and simple) bondings of a Carbon atom.
Besides this sp3-hybridisation exist another two options:
- The first (just explained above) is the hybridisation of a 2s-orbital with three 2p-orobitals to four new orbitals of the type sp3
- Now the second option is that one 2s- and not three, but only two 2p obitals participate in the hybridisation.
Then three new orbitals are made of the type sp2
and one old 2p-orbital remains unchanged; that one can take care of an extra bonding.
(double bond: 1 bonding is of the type σ and one bonding is then π).
- The third option is that one 2s-orbital and one 2p-orbital participate in the hybridisation.
Then two new orbitals are made of the type sp and two old 2p-orbitals remain;
thus a triple bond can be made between two C atoms (one bonding σ and two of π).
Normally the number of bondings per C atom is 4 (bonds of the type σ they are aliphatic).
In benzene and similar substances, every C atom had three orbitals of the type sp2
that per C atom forms three σ-bondings.
In that case, per C atom in a benzene ring is one 2p-orbital and in total 6 per ring.
They overlap in such a way that they stabilize the molecule with a very special 'fourth' bonding of the type π (aromatic).
Study the following strip drawings.
the Hydrogen atom, very different from the Carbon atom, is the most simple atom with only one electron.
The electron division is: 1s1.
Nothing to hybridise here. The s-orbital has a round shape and can overlap with any other type of orbital of whatever other atom (in carbon chemistry mostly a Carbon atom)
This overlap always gives a σ-bonding and always delivers only a simple bond.


