galvanic element
Below you find a scheme of a simple voltaic cell with copper and zincum electrodes.
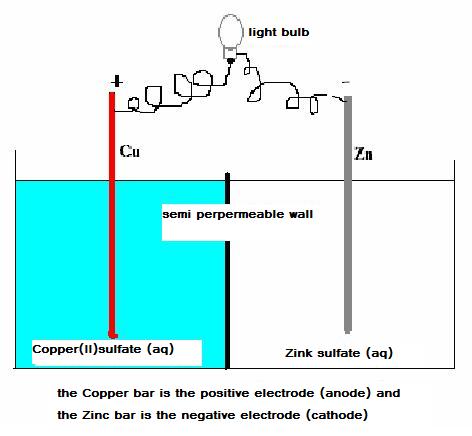
In this complex, we can discover a sufficiently strong oxydator (Cu2+) and also a reasonable strong reductor (Zn)
The spontaneous reactions that dominate the process, are:
Zn(s)  Zn2+ + 2e-
Zn2+ + 2e-
and
Cu2+ + 2e-  Cu(s)
Cu(s)
