Oxydation number
The oxydation number is the number of electrons that
- completely or partly -
is donated or captured by one or another particle.
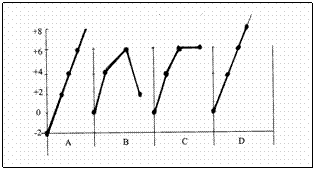
The above diagram shows the changing oxydation numbers of Sulphur during the production process of sulfuric acid.
The process can roughly be described as follows:
S  SO2
SO2  SO3
SO3
 H2SO4
H2SO4
Try to analyse that graphic D is the only one that shows the oxydation numbers in the right way.
Att.:
- Neutral elements always get an oxydation number with vaulur 0 (the atoms have not lost or gained any electron).
- Hydrogen is neutral, so has an oxydation number 0, but in compounds it is almost always +1.
- Oxygen as an element has oxydation number 0, but in compounds mostly is -2.
