Reaction diagrammes
(Bio)catalysis
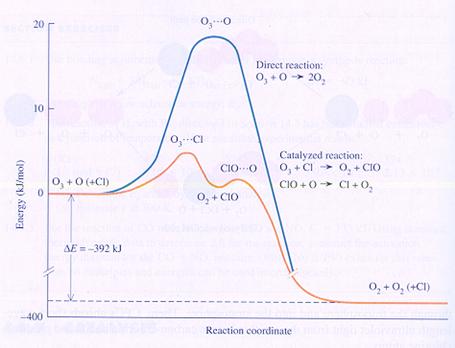
In the diagramme you can see the chemical energy on the vertical axes and the course of the reaction on the horizontal axes:
Ozon + atoms of Oxygen normally form Oxygen, with help of the catalyst Clorine atoms.
Without clorine their is the need of an intermediate product with much energy (O3---O); the formatiuon of that one costs a lot of energy.
The atom of clorine can make a different intermediate product that costs less energy.
Temperature
In general you may say that the increase of temperature will increase the reaction rate.
Every 10 degrees increase will double the reaction rate.
(kind of general rule)
Inrease of temperature means automatically: more movement of the present particles;
and this means more collisions between the reacting particles and more energy for each.
Concentration; adding or removing substances
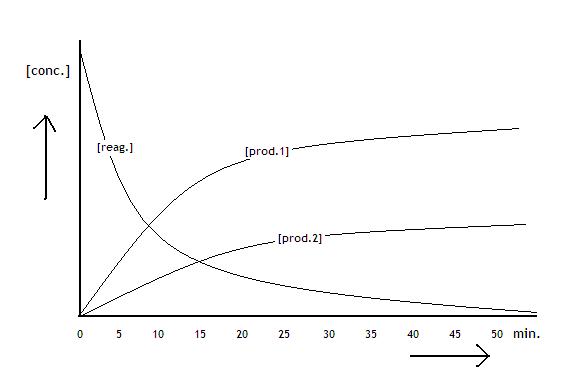
The following diagramme shows the concentrations of the reactants and products of a process that is about to reach equilibrium: