Reaction Mechanism / effective collisions
Every reaction has a reaction mechanism:
The sequence of the different reactions steps.
The slowest step determines the total, the overall reaction rate.
There are chemical reactions where the particles (e.g. molecules) - simply by possessing sufficient energy - decompose in a decomposition reaction.
In this case the reaction rate does not depend on the presence of other particles.
Such reactions are unimolecular, first order reactions: the particles do not need any collision with other particles to start a reaction. They can split spontaneously into different parts.
The decomposition of 14C is a first order reaction and extremely slow.
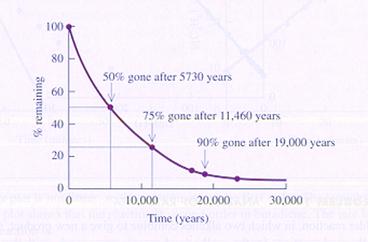
Another example of a unimolecular reaction is the decomposition of N2O4.
With sufficient energy, the molecule will divide in two parts (NO2).
This very elementary reaction consists of only one step.
The above mentioned decomposition reaction is the opposite of the reaction in the following figure:
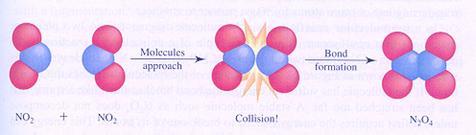
effective collisions
Most chemical reactions are not unimolecular, but bimolecular: two particles must collide, and an effective collision leads to a reaction.
These are not elementary reactions; they have a complicated reaction mechanism with various steps.
A bimolecular reaction occurs not automatically in every collision. The collision should be 'effective'.
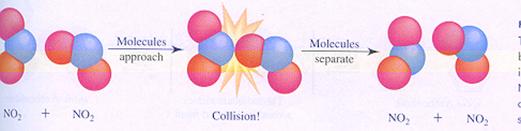
a non effective collision
Below you see the images of two possible (different) mechanisms of the reaction between molecules of nitrogen dioxyde. You see the different steps.
In case I, two molecules collide; thats how the reaction starts.
In case II, one molecule splits and one of the products collides with another molecule.
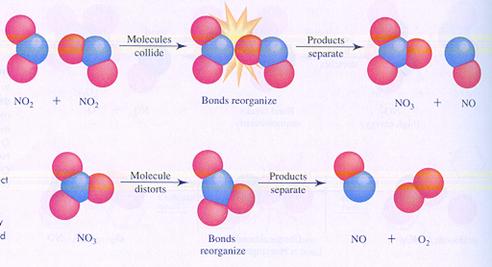
case I
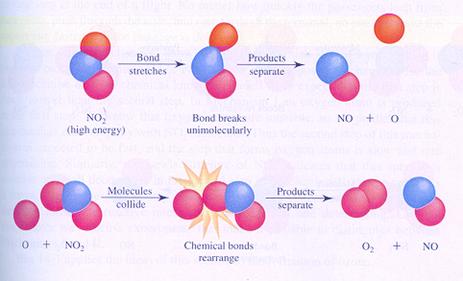
case II
The reaction rate can be influenced with different factors / conditions.
For the rate, a formula has been derived where the most important and most influencial factors are included.
The rate depend on the concentration of the reactants [ ], on the division / surface of the substance (homogeneous or heterogeneous), on the temperature (oC or K), and on the possible presence of a catalyst.
V ≈ [concentration] x division x energy of the particles x catalyst.
As such, this formula is rather useless. It is a formula in words and we want numbers.
We can make the formula more simple by keeping some conditions constant (unchanged) to stay with the (variable) concentrations.
Now we get a mathematical formula:
V = k.[conc.]n
- V: is the reaction rate;
- k: inlcudes the total of constants from the other factors / conditions;
- n: is the coŽfficiŽnt of the reactants in the reaction equation;
- The concentrations of any homogeneous substance appears in the formula. Leave out heterogeneous substances.
- A reaction with a high value for k has 'strong reactants'.
- A reaction with a low value for k has 'weak reactant'.
Important in reaction rates is the reactivity of particles. There are particles with a very high reactivity, for example the so calles 'radicals'.
Radicals are formed - generally spoken - under influence of light and have as a special property that the possess one or more single (unpaired) valency electrons.
Radicals are neutral.
Examples: Cl· Br· — C — C — O·
Other attacking particles are those with charges. Some charged particles (+, -, δ+ or δ-) may show big reactivity.
We distinguish here two possibilities:
- a negative particle attacks a positive particle = nucleophylic mechanism
- a positive particle attacks a negative particle = electrophylic mechanism
An example to investigate is the organic reaction between alkanol and alkanoic acid in the presence of the catalyst sulfuric acid.
propanoic acid + ethanol
 ethylpropanate + water (sulfuric acid is catalyst)
ethylpropanate + water (sulfuric acid is catalyst)




