We look in the table and must conclude that water is an oxydator as well as a reductor (in both cases very weak)
But Sodium is extremely strong reductor, so it makes sense to think that water in this case will act as an oxydator.
The reductor has a lower position than the oxydator, so the reaction is spontaneous.
| Red: | Na | Na+ + e- | |x2 | |
| Ox: | 2H2O + 2e- | H2 + 2OH- | |x1 |
NB: the number of electrons in both half reactions must be the same.
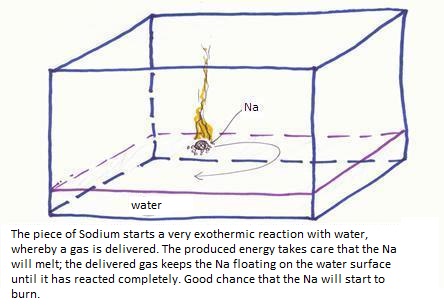
2Na + 2H2O
 2Na+ + H2 + 2OH-
2Na+ + H2 + 2OH-
of:
2Na(s) + 2H2O(l)