Methods of separation
The proper method to separate a mixture of substances into its components depend on the character of that mixture and of the components.
For example, its of no use to filtrate a homogeneous mixture.
To chose the best method, you must know the properties of the substances.
1 Extraction of (s)+(s)
Extraction of a solid mixture means: adding a solvent in which one of the components will dissolve and the other not.
Then you can separate the solution form the remaining solid, by starting the filtration.
Finally you can - if desired - evaporate the solvent and stay with the solid component.
This way hot water can extract certain substances from thea leaves. You separate components from each other and the soluble components are consumed.
The remaining substances (what's left from the leaves) is thrown away.
A mixture of Iodine and Potassium permanganate kan be separated by adding alcohol. The Iodine dissolves, but the salt does not.
And what about making coffee?
Some special extractions are:
- when heating the same mixture as above (Iodine + permanganate (= (s) + (s)) the Iodine will evaporate.
But immediately at some cooling, the Iodine will sublimate at the walls of the device (use a glass funnel and you will observe the Iodine cristals coming back, and separated from the salt cristals.
- From a mixture of Iron and Sulphur powder, you can 'extract' the Iron with a magnet.

2 Absorption bij (l)+(l) or (l)+(s) or (g)+(g)
Chromatography often is applied in chemical labs:
a mixture (meaning with different particles) passes a fixed, solid layer (paper, Aluminium oxyde gel, of other porous substances)
The different particles of the dissolved mixture are more or less strongly absorbed by that fixed layer (dependent on, for example, polarity)
touch a piece of filter paper in black ink (that for sure is a mixture of various colorants). That piece of paper shows a small black spot or tiny black horizontal line.
Chose a 'solute' and put the paper with its lower side into that solute. By capillarity, that solute will go up in the paper and also pass the ink spot. At that very moment the solute will take the components up, with the solute and with different rates.
That's how the most bad solubel component will remain at its original place, will not move up.
But another component that dissolves reasonably in the solute, will go up with that upmoving solid.
That's how you separate the components. At the end you can observe different spots with different colors at different places.
3 Electrophoresis and Chromatography
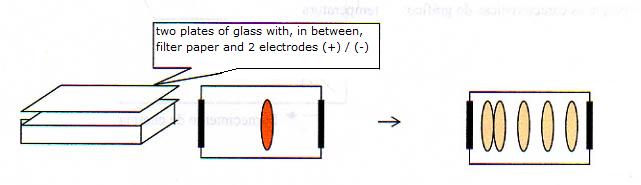
A special filter paper is wetted with an aquaous solution, maybe acid, basic or neutral.
In the middle you put a spot of a protein mixture.
The proteins move under influence of an electric field, and according to their charge, in a certain direction:
- proteins in basic environment (OH- is present) mostly have a negative charge
- proteins in acid invironment (H+ is present) mostly have a postive charge
- the proteins in their iso electrisch point (more about that later!) are neutral
The result that can be seen in the image indicates that probably an acid liquid was used to wet the filter paper.
There are different chromatographic methods like:
- paper chromatography
- column chromatography
- gas chromatography
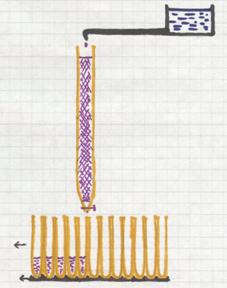
About the special type of chromatography, the column chromatography.
The column (a glass tube) is full of a special solid (sand, Aluminium oxyde, a certain gel, and others)
On top of the column content is placed a mixture that just will be absorbed by the content (gel) and immediately after that, you open a tap and drops of a solute are provided to the column. This solute goes through the column and will take the mixture with it, every component at its own rate. That rate depends on factors like:
how big are the particles of a component? how soluble is the component in the solute? how well is the component absorbed at the solid content of the tube? etc.
Every five or ten seconds a new test tube is places under the column to receive the outcoming drops. At the end of this process, every test tuybe contains a bit of the solute, and some tubes will contain certain amount of a component.
There are very good detection methods to find out what component is in every tube.
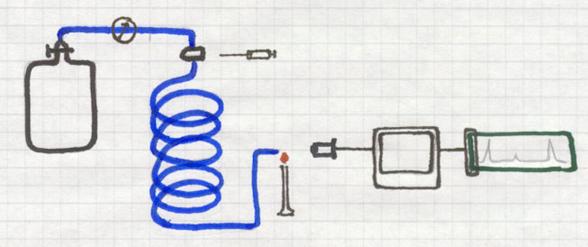
Gas chromatography also is a method that applies absorption.
Substances will be absorbed by a 'carrier'.
A long, thin, spiral shaped tube is full of a porous solid, the carrier.
Continuously an (inert) gas is passing that tube, or better: the carrier.
That gas could be, for example, He, coming from a cylinder where the gas pressure can be controlled very well. Helium is one, but there are other inert gases.
At the beginning of the tube there is an injection room where a gas mixture can be injected. That unknown gas mixture is to be investigated.
The injected small amount of gas mixture immediately is taken with the carrier gas He all through the tube and the solid carrier.
One component will move faster that the other, dependent on its absorbtion by the carrier. So one gas will leave the tube earlier than the other.
At the end of the tube, for example with a micro flame that react with color change, the outcoming component will change that color.
A detector can analyse that color as well as the intensity of the color, and give a signal that can be printed out.
Thus you cannot only determine which component was present in the mixture, but also its percentage.
3 Distillation of (l) + (l)
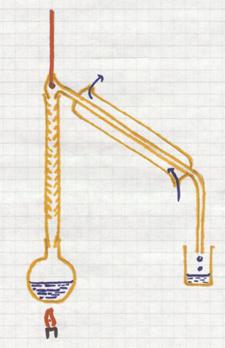
This is a separation method based upon the difference in boiling points of liquids.
If you heat a mixture of liquids, the liquid with the lowest boiling point will boil first, escape first (evaporate) from the mixture.
The vapor enters the condensation tube with cooling. There this component will condens and collected.
The other components, with higher boiling points, remain behind until reaching higher temperatures.
Good controll of the temperature is needed during the whole process.
example: you can distill wine, where alcohol with the lower boiling point (78ºC) will escape. the water will remain behind
4 Filtration of (s) + (l)
Filtration is only possible with heterogeneous mixtures, and with particles whos size is big enough for the filter. They cannot pass, but the (smaller) liquid particles can.
Filters exist in all kinds, from very fine to very rude.

5 Precipatation at (s) + (l)
You clearly have a solid in a liquid, a heterogeneousmixture, and the precipitate(s) has reasonable heavy cristals.
Then you can start to 'decantate' = let the liquid go while the solid remains behind.
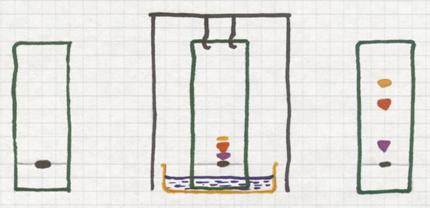
6 Evaporation of (l) + (s)
Normally we deal here with homogeneous mixtures. Otherwise you would filter or decantate.
You heat such a mixture carefully. The liquid evaporates and the solid remains.
Of course only when the liquid and the solid can stand heating without decomposing.

It deals with a homogeneous mixture of (l) + (s)
The image shows a method to evaporate carefully (on boiling water in a water bath).
Such a water bath can only serve as such if a temperature of 100ºC is enough to evaporate the liquid.
The dissolved substance will remain behind.
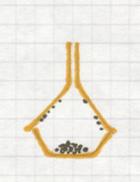
7 Centrifugation of (s) + (l)
Centrifugation can only be done with heterogeneous mixtures. Often it is chosen as a faster method than filtration.
The particles with the biggest mass (that must be the solid) will precipitate at the bottom of the tube under influence of centripetal forces.
You apply this method for example when you have a suspension: a trouble mixture of (s) + (l) that could not be separated with a filter.
The solid could be built up os very tiny cristals or even macromolecules.
There are methods for centrifuging (g) + (g). Gas centrifuges are applied to separate, for example, heavy Hydrogen from normal Hydrogen.









