Redox in Carbon chemistry
Also in the carbon chemistry there are redox reactions. Many carbon compounds are capable to donate or capture electrons.
att.: talking here about oxydation, we do not actually talk about combustion with Oxygen.
Most compounds in carbon chemistry can suffer combustion and form carbon dioxyde and water, but this is not now the topic.
But yes, I give you first a short info about direct and indirect combustion; after that we go to the real redox reactions.
Complete combustion
A nice example of a complete combustion (although in steps) you can find in question 39.
Here is already the reaction:
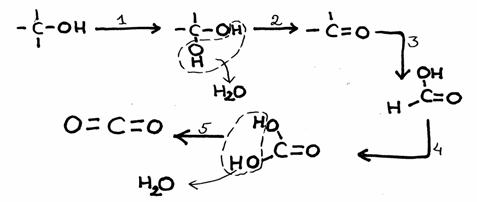
Most Carbon bondings (with C and H and other elements) react (connect) strongly with Oxygen.
The most reactive element is here Hydrogen that immediately - in contact with Oxygen - creates water.
Then (but almost simultaneously) the Carbon reacts and forms CO2.
Possible present S or N or P or other elements can make more products like SO2 and the Nitrogen oxydes NxOy.
These (gaseous) products are responsible for air pollution.
Of course, the best petrol contains as less sulphur and nitrogen as possible.
Incomplete combustion
Takes place is there is insufficient Oxygen.
Of course H is the first to produce water, but maybe there is not enough Oxygen left over to burn the carbon atoms.
There might be insufficient air, for example.
Then CO (Carbon monoxyde) is formed, or even, the carbon remains unburned (soot).
So take care that you always provides the burning process with enough air.
Carbon monoxyde is very toxic.
