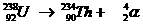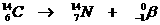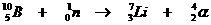The reaction equation
Changes during a chemical reaction are indicated with an arrow:  ,
the reacting substances before, de producst after.
,
the reacting substances before, de producst after.
Reactants
 Products
(reaction equation)
Products
(reaction equation)
sometimes you can write a reaction in words (like: Calcium with water  Chalk and Hydrgen),
Chalk and Hydrgen),
but normally we use the chemical symbols for each participating particle and we show the right mol proportion as also the phase of each component.
The total number of participating elementary particles (atoms and ions, but also protons, neutrons and electrons) does not change during the reaction.
You start a reaction of course with the right formulas; but if they are there, you must take care that the number of particles per element is equal at both sides of the arrow. No atoms disappear, nor are created during a normal chemical reaction.
With 'coefficients' we 'balance' the equation: for example, 2 molecules of this react with 3 ions of that, etcetera.
These coefficients show us: the mol proportion.
Besides that, you always must have a look at the electric charge at both sides.
That also may not suffer any change.
If you notice any charge change in the equation, something is wrong.
In general:
- You take care of the right formulas of the participating substances;
- You balance the equation with coefficients;
- You add the phase of every substance: (s) (l) (g) (aq); [(aq) is in fact not a real phase!]
- Of ions you write normally the ionic formulas in the equation (but not always!)
Only if calculations are to make (reaction calculations), than it is advisable to write the empiric formulas (not de ionic), for the substances built up of ions.
Very special is the nuclear reaction. There the elements change, what never happens in normal chemical reactions.
Yet the normal rules are to be applied in nuclear reactions: number of particles and charge at both sides of the arrow.
Examples: