Adding or withdrawing heat energy in a chemical equilibrium
Adding extra energy to a chemical equilibrium will also result in an attempt to counteract this change.
In this case the equilibrium could try to consume that extra added energy, or: stimulate the endothermic reaction.
In the case of HI the equilibrium will dislocate to the left. The forward reaction is exothermic.
[glucose + fructose  saccharose + water ΔH < 0]
saccharose + water ΔH < 0]
The saccharose-equilibrium is exothermic to the right.
At lower temperatures, less energy will be available and the equilibrium will try to 'produce' extra energy.
This can be realised by stimulating the exothermic reaction, the one to the right.
Or: at lower temperatures, more saccharose will be formed.
All what was said above is a qualitative view.
Youi can also investigate the same effects in a quantitative way, and prove it with the equilibrium constant K.
That K has a fixed value and does not change, whatever you change at the equilibrium (exept temperature).
Those proves can be delevered with mathematical calculations.
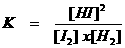
Imagine that the amount of I2 is increased.
Look at the mathematical formula for K. If the [I2] increases and K may not change, then, in a mathematical view, the amount of HI must also increase, or: the equilibrium must dislocate.