Kw and the autoprotolysis of water
In water solutions always the water molecules are present in incredible amounts and they continuously have contact.
Every watermolecule has a (weak) tendency to donate and to accept protons; this is possible when two water molecules meet:
| H2O |
+ |
H2O |
 |
H3O+ |
+ |
OH- |
|
ΔH |
> 0 |
|
|
|
|
|
|
| | | |
Make notice of the fact that both ions are produced in equal amounts.
The water equilibrium can only exist in watery environment, and is dislocated very far to one side (the side of the not dissociated molecules), and few ions.
The equilibrium constant will have a low value.
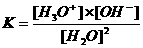

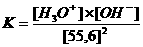

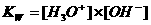
In neutral aquaous solutions at 25ºC, the following will happen:
Of every MOL water molecules (being 6 x 1023) "only" 6 x 1016 have caught a proton and simultaneously, the same amount have donated a proton.
In water of 25ºC the concentration [H3O+] = [OH-] = 10-7 mol/dm3
or:
pH = pOH = 7
Kw (the constant of the autoprotolysis) = K x (55.6)2 = [H3O+] x [OH-] = 10-14 mol2/l2
The autoprotolysis of water is an endothermic process.
Pure boiling water (so at a temp of 100 oC) has no pH=7"