Mass number & atomic mass
There is something that can difficult the calculations with those concepts, and that is the existance of isotopes.
for example: Of the element Chlorine do exist two types of isotopes in nature: Chlorine atoms with 18 neutrons and Chlorine atoms with 20 neutrons.
Of course every atom of Chlorine has also 17 protons.
1 MOL of the isotope 35Cl has a mass of 35 g [atomic mass = 35]
1 MOL of the isotope 37Cl has a mass of 37 g [atomic mass = 37]
In nature exists a mixture of two isotopes in the proportion of (±)3 : 1
which means that the element Chlorine, as it occurs in our world, has an average atomic mass of about 35,5
Something like that is the case with many elements. That's why in practice most atomic masses of the elements are not exactly the same as the mass number of one isotope of that element. In tables you find always the averages.
Molecules can differ in size. Water has very small molecules and proteins are enormous macromolecules.
Amilose also is very big; it is a polyglucose, built up of many connected molecules of glucose.
It is not easy to imagine the size of one particle of a substance; it is incredible small.
Yet there are (relative) big differences between those particles: like starch, having macromolecules, so big that the water in which the starch was dissolved, turns cloudy/trouble. The smaller sugar molecules will never cause this outlook. A sugar solution always will be a transparant mixture, like most solutions in water.
And about solids and liquids, in practice you can observe them, even knowing that the particles are so tiny. They are so close toghether that we can see the collection.
But you need an enormous number of particles before they are visible as a substance.
Gases can only be seen if the molecules are colored.
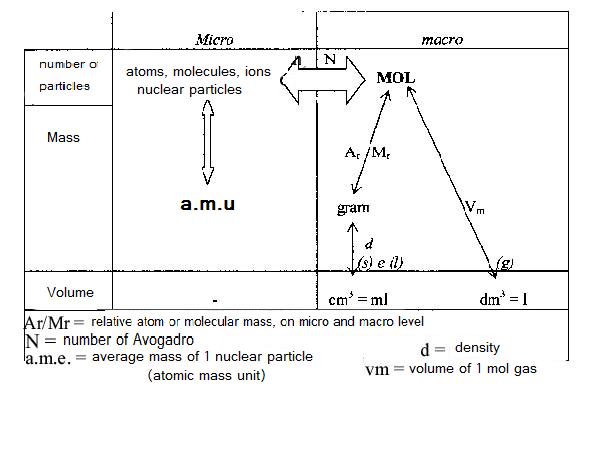
The diagramme below shows the relation between the units on micro level (atoms etc) and on macro level (grammes etc.). The unit MOL is found in the centre of the scheme:
Example 1:
4 grams of the substance CuSO4.5H2O is weighted.
How many MOLs of hydrated Copper sulphate (light blue cristals) is that?
Use tabel V of the table book.
answer:
From grammes to MOL you must use the molecular mass. In this case: Cu + S + 9O + 10H = 63.5 + 32.1 + 144 + 10 = 249.6
249.6 gram of the substance (hydrated Copper(II)sulphate) = 1 MOL.
 1 gram = 1/249.6 MOL
1 gram = 1/249.6 MOL
 4 grammes = 4 x 1/249.6 MOL = 0.016 MOL
4 grammes = 4 x 1/249.6 MOL = 0.016 MOL
4 grammes of CuSO4.5H2O equals 0.016 MOL CuSO4.5H2O
or:
0.016 MOL CuSO4.5H2O weights four grammes.
Example 2:
Suppose that a glass of beer (say: 100 ml) contains 5 ml of alcohol (C2H5OH, density of alcohol = 0,8g/ml)
How many grammes of alcohol are found in one liter of beer?
Answer:
We already know the number of ml, but the question is to find grammes of a liquid (alcohol)
so you understand that we will need the density. d = 0,8 e.g.: 1 ml of alcohol has a mass of 0,8 gram.
 5 ml weights 5 x 0,8 gram (= 4 grammes).
5 ml weights 5 x 0,8 gram (= 4 grammes).
One glass (=100 ml beer) contains 5 ml = 4 gr alcohol
 1 liter (=1000 ml) must contain 10 times more = 40 gr of alcohol [that is enough to slow down the growth of brain cells!!]
1 liter (=1000 ml) must contain 10 times more = 40 gr of alcohol [that is enough to slow down the growth of brain cells!!]
