KS Solubility product
Salts can dissolve in water well or badly, meaning that many of few free ions are produced, given to the water.
The ions of the dissolved salt will reach a certain (maximum) concentration.
The mathematical product of these ion concentrations we call: the Ions product (Ip).
About the above mentioned ions and the ions product: we have to deal with an equilibrium as soon as badly soluble salts are involved.
Example:
Silver chloride is an insoluble salt (better: badly soluble salt). Totally insoluble salts do not exist. There is always a small amount that dissolves in water
There is always the opportunity for ions in a lattica (at the outer side) to escape and to move into the water.
If they cannot all be set free, because the lattice is too strong, then at the end you reach a situation of equilibrium, where the free ions are in dynamic equilibrium with the lattice. They escape, but as soon as positive and negative ions meet, they join the lattice.
AgCl(s)  Ag+(aq)+ Cl-(aq)
Ag+(aq)+ Cl-(aq)
After reaching the equilibrium, and if we keep the temperature constant, the concentrations will no longer change.
(att.: the solid AgCl is heterogeneous and has no concentration (=1)).
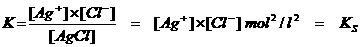
Thus every salt with a small solubility will have a KS, called: Solubility product
Imagine you dissolve a tiny littlebit of a badly soluble salt. First there will be an increasing ions product, but soon the maximum value of the Ip will be reached: then the ions product is equal to the solubility product.
Every tablebook has a table with solubility product of salts.
Here we have a table that shows if a salt is more or less soluble.
The salt(s) will reach an equilibrium with its ions(aq) and then the concentrations of the ions will no longer change. The solution has become "saturated": At this temperature no more salt will dissolve.
The ions product has reached its maximum value = the solubility product.
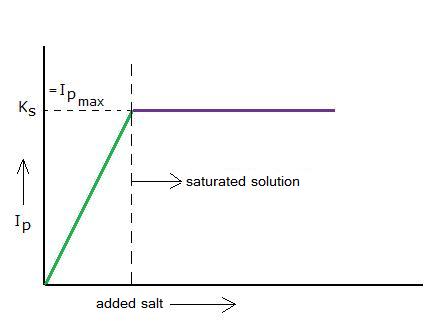
N.B. Do never confuse the solubility product with the actual solubility of a salt!
The solubility is the number of Mols of that salt that will dissolve per liter water (depends on the temperature).
The KS is the product of the ion concentrations of the salt in a saturated solution.
